Our Research
Understanding the intricate interplay between cell proliferation, the cell cycle, and cellular differentiation is critical to unravelling the fundamental biology of multicellular organisms. Our research tackles this fundamental question by utilising the genetics of the multicellular model organism Drosophila melanogaster, together with advanced in vivo imaging, biochemistry, and multi-OMICs techniques. Dysregulation of these processes can disrupt normal embryonic development and adult tissue homeostasis and has been implicated in various human diseases, including cancer, fibrosis, and neurodegenerative disorders. Through our work, we aim to uncover the evolutionarily conserved molecular mechanisms that integrate the cell cycle and cellular differentiation in metazoan organisms. Additionally, we employ human cell culture and mammalian organoids to translate our findings in flies to humans, with the ultimate goal of improving human health through our discoveries.
理解细胞增殖、细胞周期和细胞分化之间复杂的相互作用对于揭示多细胞生物的基本生物学至关重要。我们的研究通过利用多细胞模型生物果蝇(Drosophila melanogaster)的遗传学,结合先进的活体成像、生物化学和多组学技术来解决这一基本问题。这些过程的紊乱可能会破坏正常的胚胎发育和成体组织稳态,并与各种人类疾病有关,包括癌症、纤维化和神经退行性疾病。通过我们的工作,我们旨在揭示在后生动物中整合细胞周期和细胞分化的进化保守分子机制。此外,我们还利用人类细胞培养和哺乳动物器官样本来将果蝇中的发现转化为人类,最终目标是通过我们的发现来改善人类健康。
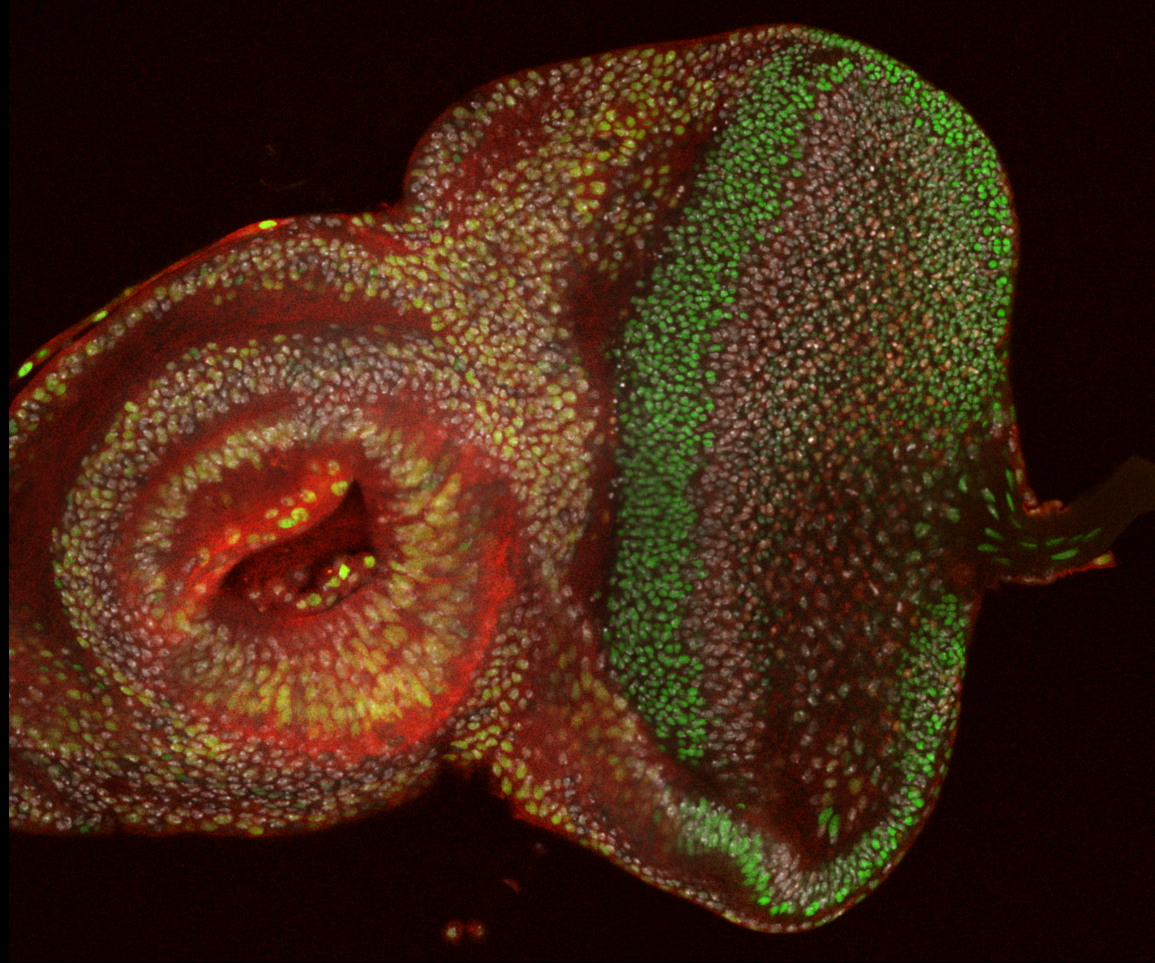
Exploring the Roles of Cell cycle regulators in Cell Fate Decision and Differentiation 探索细胞周期调节因子在细胞命运决定和分化中的作用
Cell cycle regulators (CCRs) are well-known for their crucial role in controlling cell cycle progression. However, recent research, including our own, has revealed that some of these CCRs also have a direct impact on the cell fate decision and differentiation processes. By delving into these novel metazoan-specific functions of CCRs in various tissues of Drosophila, we aim to uncover their underlying mechanisms and roles. Additionally, we investigate their conserved roles in cultured human cells and tissues, paving the way for groundbreaking discoveries in the field of cell biology.
众所周知,细胞周期调节因子(CCRs)在控制细胞周期进程中起着关键作用。然而,最近的研究,包括我们自己的研究,揭示了一些CCR还直接影响细胞命运决定和分化过程。通过深入研究果蝇各种组织中CCR的这些新的多细胞生物特异功能,我们旨在揭示其潜在机制和作用。此外,我们还研究了它们在培养的人类细胞和组织中的保守作用,为细胞生物学领域的突破性发现铺平了道路。
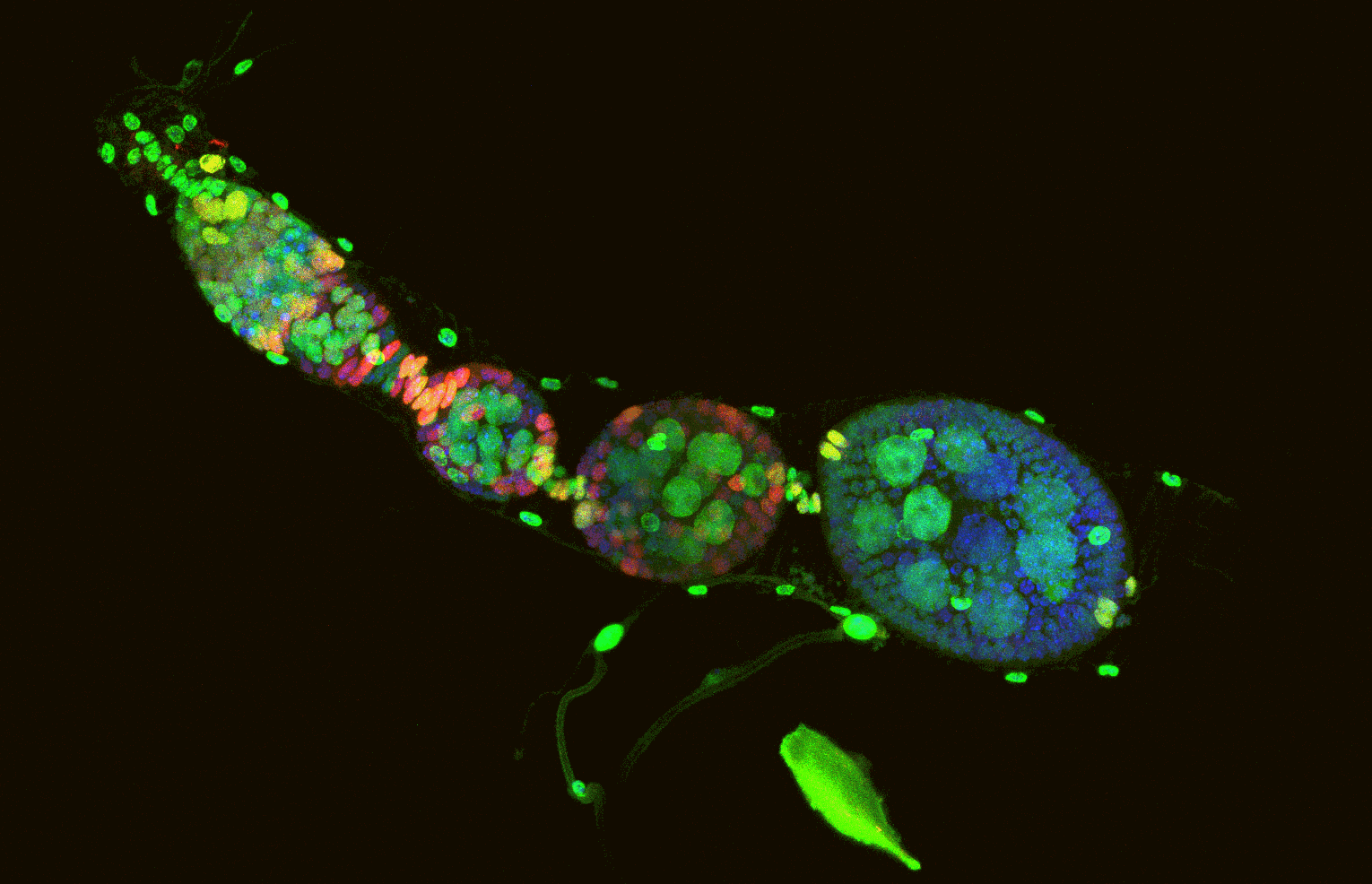
Cracking the Code of Cellular Quiescence and Cell Cycle Exit 细胞休眠和细胞周期退出机制
In the adult human body, the vast majority of cells exist in either a reversible dormant state (G0 phase) or have irreversibly exited the cell cycle. Understanding the mechanisms that regulate the entry and exit from these cellular quiescent states is crucial to unlocking the secrets of cellular biology. Through our research, we utilise the Drosophila adult brain and gut as in vivo models, as well as in vitro cultures of transformed and untransformed human cells, to gain a deeper understanding of these mechanisms. Additionally, we investigate the genetic and epigenetic changes that may occur in the genome DNA during these transitions and explore their long-term consequences on genome stability and tissue homeostasis. Our findings have the potential to revolutionize the field of cell biology and have implications for a wide range of diseases, from cancer to neurodegenerative disorders.
在成人人体中,绝大多数细胞要么处于可逆的休眠状态(G0阶段),要么不可逆地退出细胞周期。理解调控细胞进入和退出这些休眠状态的机制对于揭示细胞生物学的奥秘至关重要。通过我们的研究,我们利用果蝇成人大脑和肠道作为体内模型,以及转化和未转化人类细胞的体外培养,来深入了解这些机制。此外,我们还研究了在这些转变过程中可能发生的基因组DNA的遗传和表观遗传变化,并探索了它们对基因组稳定性和组织稳态的长期影响。我们的发现有可能彻底改变细胞生物学领域,并对广泛的疾病产生影响,从癌症到神经退行性疾病。
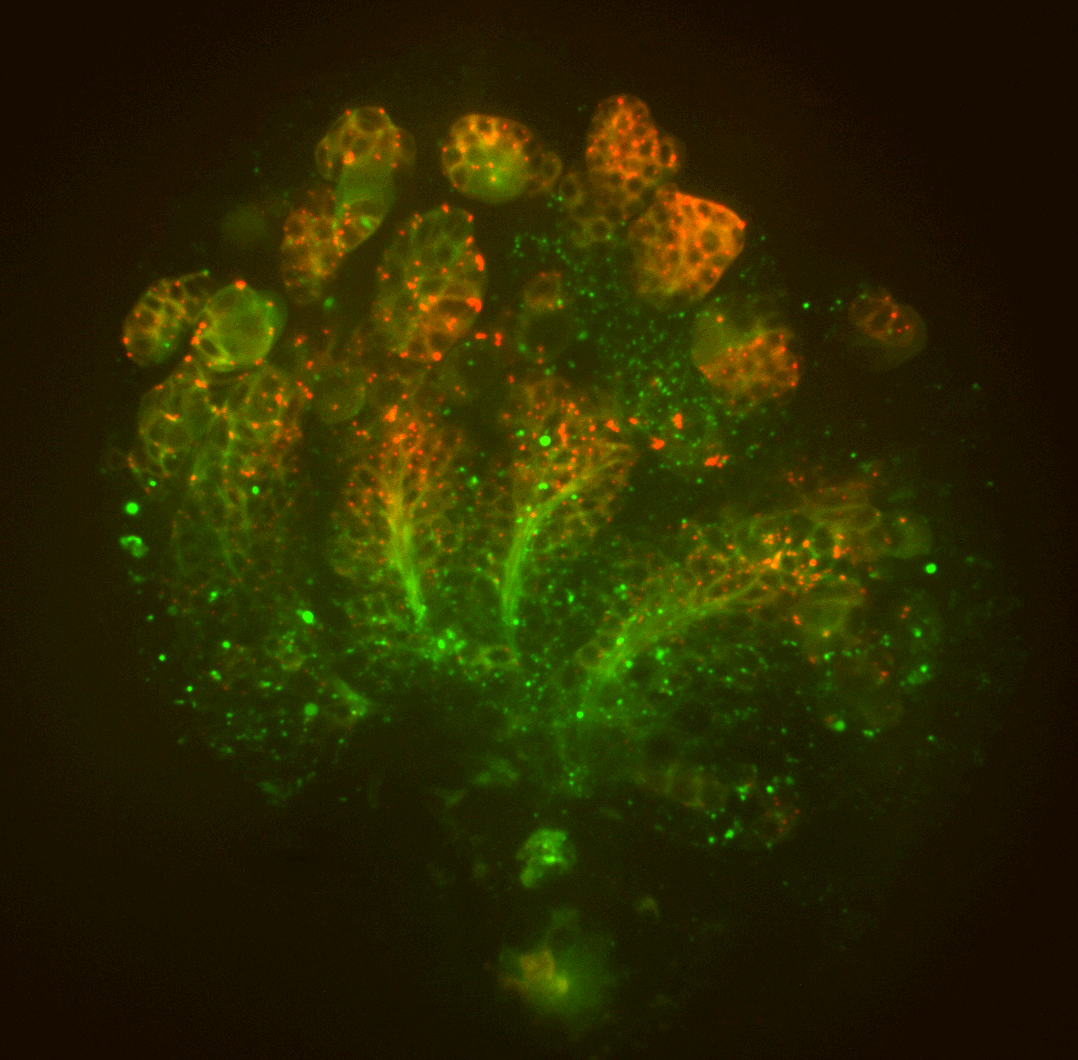
Investigating Molecular Interactions of Metazoan-Specific Organelles with CCRs 多细胞生物特有细胞器与细胞周期调节因子之间的相互作用
The centrosome and the cilium are critical membrane-less cellular structures found specifically in animal cells. These structures control a wide range of cellular events, including cell migration, cell/tissue polarity, intracellular transport, and signal transduction, by organizing cytoskeletal elements. At our lab, we investigate the molecular crosstalk that occurs between CCRs and components of these organelles. By utilizing Drosophila tissues and human cell culture, we aim to uncover the critical roles of these interactions in cell cycle control, development, and physiology. Our findings have the potential to transform the field of cell biology and have implications for a wide range of diseases, from developmental disorders to cancer.
中心体和纤毛是特定于动物细胞的关键无膜细胞结构。这些结构通过组织细胞骨架元素来控制广泛的细胞事件,包括细胞迁移、细胞/组织极性、细胞内运输和信号转导。在我们的实验室里,我们调查了CCR与这些细胞器组件之间发生的分子交流。通过利用果蝇组织和人类细胞培养,我们旨在揭示这些相互作用在细胞周期控制、发育和生理中的关键作用。我们的发现有潜力改变细胞生物学领域,并可能对广泛的疾病产生影响,从发育障碍到癌症。
Latest News
Successful 2023 ShanghaiTech Summer Camp: Shaping the Future of Research in China / 2023年上科大暑期夏令营:塑造中国研究的未来
In mid-July, our school hosted the annual summer camp, a key student recruitment event for our graduate program. This tradition has become an essential platform for connecting our school with prospective graduate students, enabling them to explore our research, faculty, environment, activities, and student life. This year’s summer camp saw around 200 promising candidates in […]
Congratulations to Amanda and June on Their Graduation Success! 祝贺张博士和夏雪的毕业成功!
On May 10th 2023, our laboratory celebrated two outstanding achievements as Amanda and June successfully defended their theses. Amanda (Zhang Qian) has become the first PhD from our lab at ShanghaiTech. She impressed us with her work on the regulation of Dpp signalling by the cell cycle regulator APC/C-SnoN, and on the role of Spd2 […]
Maite and Yuu Presented Research at the CSCB Meeting
We are thrilled to share that our lab members recently attended the China Society of Cell Biology (CSCB) Meeting held in Suzhou from April 11th-14th, 2023. This was not only a great opportunity for us to share our latest research findings, but also the first event we have been able to attend since the beginning […]
The Team








Funding
We thank ShanghaiTech University, National Natural Science Foundation of China (NSFC), Shanghai City Science Committee, Cancer Research UK, BBSRC and the European Commission for their current and previous support for our research.